SAS® Savvy
"Good idea, and the website looks good.", Kriss Harris, Computational Statistician
Pharmaceutical SAS® Programmer Topic Preview
Successful FDA submissions require optimal project management skills as well as productive tools. Generally all three basic constraints need to be planned across each of the four main topics (CDISC SAS Datasets, Tables, Lists and Graphs, and Validation): scope, schedule, and budget.
SOP: Program Index File >>> Variable Attribute File >>> Tables and Lists
General Macro Utility: Clinical Data Management: Building a Dynamic Application, Art Carpenter, Richard Smith
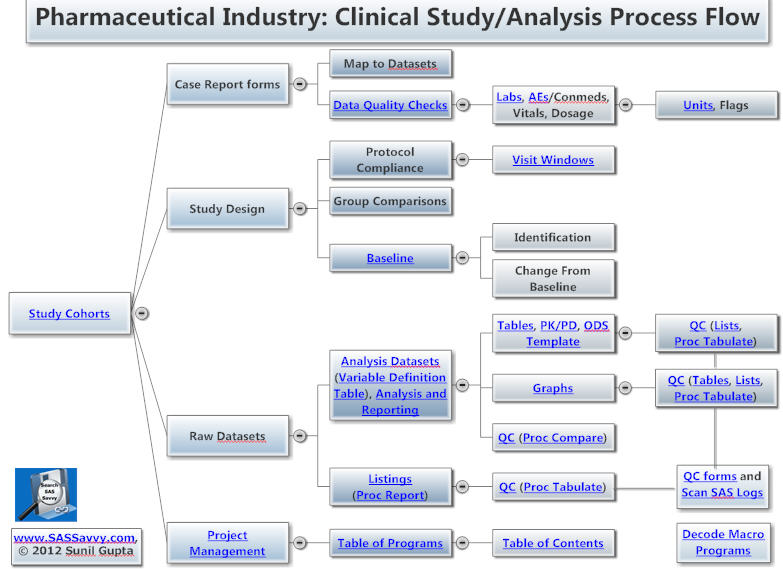
(Click on image below to start Pharmaceutical Industry mind map)

Top SAS® Programming Papers
1. Standardizing Data Processing and e-Publishing for the Pharmaceutical Industry, Shawn Wang
2. Get your SAS in gear – Automate the Production of Analysis Datasets, Liz Taylor
3. Get your SAS in gear – Automate Appendix Numbers, Titles and Footnotes, Liz Taylor
4. Auto-Attributes: No More Typing of Variable Labels (and Other Attributes)!, Jonathan Squire
5. Developing, Managing, and Evaluating a Standard Macro System, Albert Mo
6. Using Metadata for Data Driven Programming, Brian Varney
7. Transforming Business Requirements into System Solutions, Yongcun Zhang
8. Supporting the Program-Analyze-Write-Review Process with a Development
Environment for Base SAS and the Macro Language, Barry Cohen
9. Data Savant Consulting Macros
10. Names, Names, Names - Make Me a List, Ian Whitlock
11. Designing Clinical SAS Service Request Forms, Sunil Gupta
12. Utilizing Clinical SAS Report Templates, Sunil Gupta
13. Sending Emails in SAS to Facilitate Clinical Trial, Frank Fan
14. How to Write Standard Operating Procedures, Lori Hardwick
15. Ten Ways to Improve the Efficiency of Clinical Statistical Programming, Amos Shu
16. Rethink Training - Four reasons your FDA compliance training isn’t preventing violations undefined and how you can change that, Ellen Leinfuss